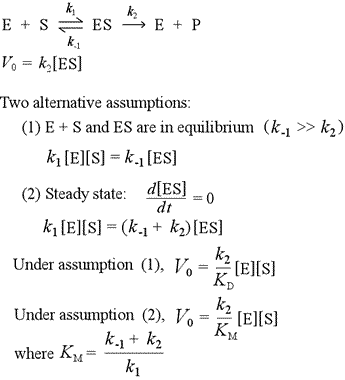
Michaelis Menten Crack+ With Key Free Download [32|64bit] [2022-Latest] Michaelis-Menten Kinetics is a very helpful tool to study and learn about enzyme kinetics. You can set the substrate concentration and the product concentration, and the app will tell you the kinetic constants. Michaelis-Menten constant is Km, the substrate concentration where the reaction rate is half of the maximum. Vmax is the maximum rate of the reaction that occurs at a certain concentration of substrate. Full Review: As you can see, this app is very easy to use. You just have to select the right reaction for studying the k constants. It's very important to be able to study the kinetics of enzymatic reactions. So, be sure to include this in your program. Our goal is to provide only the best quality apps for your device. Enjoy! More Chemistry Help is available for free at the iTunes store. The following languages are available: English Spanish French Portuguese Swedish Romanian Japanese Korean Arabic Hindi Hungarian Malay Italian Norwegian Polish Russian Serbian Finnish Greek Ukrainian Czech Slovak Danish Turkish Turkish Polish Slovenian Croatian Portuguese Czech Hungarian Slovak Czech Hungarian Slovak Croatian Portuguese Czech Hungarian Slovak Croatian Portuguese Czech Hungarian Slovak Croatian #337 I have reviewed the app, and now it is available at the iTunes App Store. It's very easy to use. Just select the right enzyme, set the concentration of the substrate, and press the calculate button. You can press the refresh button anytime and the app will update itself. The app is already available for free. However, you can download More Chemistry Help app from the iTunes store and get the free version to study enzyme kinetics. #307 I have reviewed the app, and now it is available at the iTunes App Store. It's very easy to use. Just select the right enzyme, set the concentration of the substrate, and press Michaelis Menten Crack Full Product Key Free Download The initial velocity is defined as the rate at which product is formed. This is a linear function of the enzyme concentration, so that v=rate=k[S]+[E] (or v=k[S]/([S] +[E])). If the enzyme concentration is fixed, then the rate of product formation is proportional to the substrate concentration. S. Inhibitors and Activators: Active site ligands that bind to an enzyme at or near its active site prevent the enzyme from working and can be either activators or inhibitors. These molecules will not bind to an enzyme without the help of a protein. When a substrate binds to the active site of the enzyme, it changes the enzyme's shape and creates binding sites for other substrate molecules. More substrates will bind more easily than one. Inhibitors and activators can bind to the enzyme and prevent the enzyme from working. If a good substrate cannot bind to the active site, then it will not be converted into product. Even if the enzyme can bind to a ligand that is not a good substrate, the ligand will not activate the enzyme. The equilibrium constant for a reaction is defined as the ratio of products to substrates in the absence of enzyme. K = P[S]/P[E]. Note that if K = 0, then the reaction will never go to completion. Although the products and substrates will form the same amount of molar concentration, their concentrations are in the ratio of products to substrates. This will be a very important point when we study the equilibrium between product and substrate. If we rearrange the equation to the new form, we can see that K = [E] /([S] +[E]). In most reactions that enzymes catalyze, the enzyme binds to the reaction first. The enzyme that we will be studying will have a very small concentration in solution and there will be no substrate present at first. For this reason, the process that we will study will be denoted as forward reaction. This will be the base case for studying the equation. If we want to study the reverse reaction, we will have to make some assumptions about the concentration of enzymes and substrates and check that the equations and graphs are the same. More Chemistry Help: If you do not have the More Chemistry Help app, you can visit our home page and download the app for free. In this program, we will be using the reactants and products at equilibrium to learn more about the kinetics of an enzyme. The forward reaction is the base case. In the base case, we will have 0 enzyme present in solution, so that the concentration of the substrate is 0. We will start with substrate, S, at 20 mM. There are two problems to consider when choosing the substrate concentrations for the base case of the reaction. The first is the maximum concentration of the substrate that can be dissolved in the buffer 8e68912320 Michaelis Menten Crack + Free This program is for people who study enzymology. The program allows you to calculate the rate of a reaction at different substrate concentrations. Once you are happy with the results, you can export the data to an excel spreadsheet, or read in your own data to calculate it. To find out more visit the program's home page. How to open the program: - Download and run the zip file. - Open the main window. - Click on the 'Start' button. - Enter the data you would like to use for the calculation in the 'Data' tab. - Click on 'Calculate' button. - The results will be displayed in the 'Calculated' tab. - The data in the 'Calculated' tab can be exported to a spreadsheet by clicking the 'Export' button. - The data can be read in to calculate your own data by clicking the 'Read' button. - Features: - Allows you to calculate the Michaelis-Menten parameters for your own data. - Allows you to use data from any experiment performed in the past. - Allows you to study the kinetics of reversible reactions. - Allows you to study the kinetics of irreversible reactions. - Allows you to study the Michaelis-Menten parameters of mixed-type reactions. - Allows you to find the Km for an enzyme or substrate and the Vmax for an enzyme. - Allows you to compare the Michaelis-Menten parameters of two different experiments. - Allows you to calculate the change in rate in relation to the change in substrate concentration. - Allows you to calculate the change in rate in relation to the change in Vmax. - Allows you to calculate the change in rate in relation to the change in Km. - Allows you to calculate the half life of an enzyme. - Allows you to change the units of your data. - Allows you to change the units of your calculated data. - Allows you to calculate the relative kinetics for two different enzymes. - Allows you to change the working units of the equation. - Allows you to solve the equation for different values of k1 or Vmax. - Allows you to calculate the kinetic constants for any enzyme you like. - Allows you to change the units of your equation. - Allows you to export your own data to a spreadsheet. - What's New In Michaelis Menten? System Requirements: Microsoft Windows 10, Windows 8, Windows 7 or Windows Vista Windows XP Intel Processor with at least 2 GB of RAM (4 GB recommended for highest performance) 2GB of free hard drive space Mouse or touchpad 1024 x 768 resolution monitor Default mouse settings 25-60 Hz Keyboard & Mouse Installation Instructions 1. Run the installer. The installer will guide you through the steps. You may optionally download the following before you run the installer: .zip
Related links: